Q 1973856746
 Calculate the atomic mass (average) of chlorine
Calculate the atomic mass (average) of chlorine
using the following data:
Class 11 Exercise 1 Q.No. 9

using the following data:
Class 11 Exercise 1 Q.No. 9

Solution:
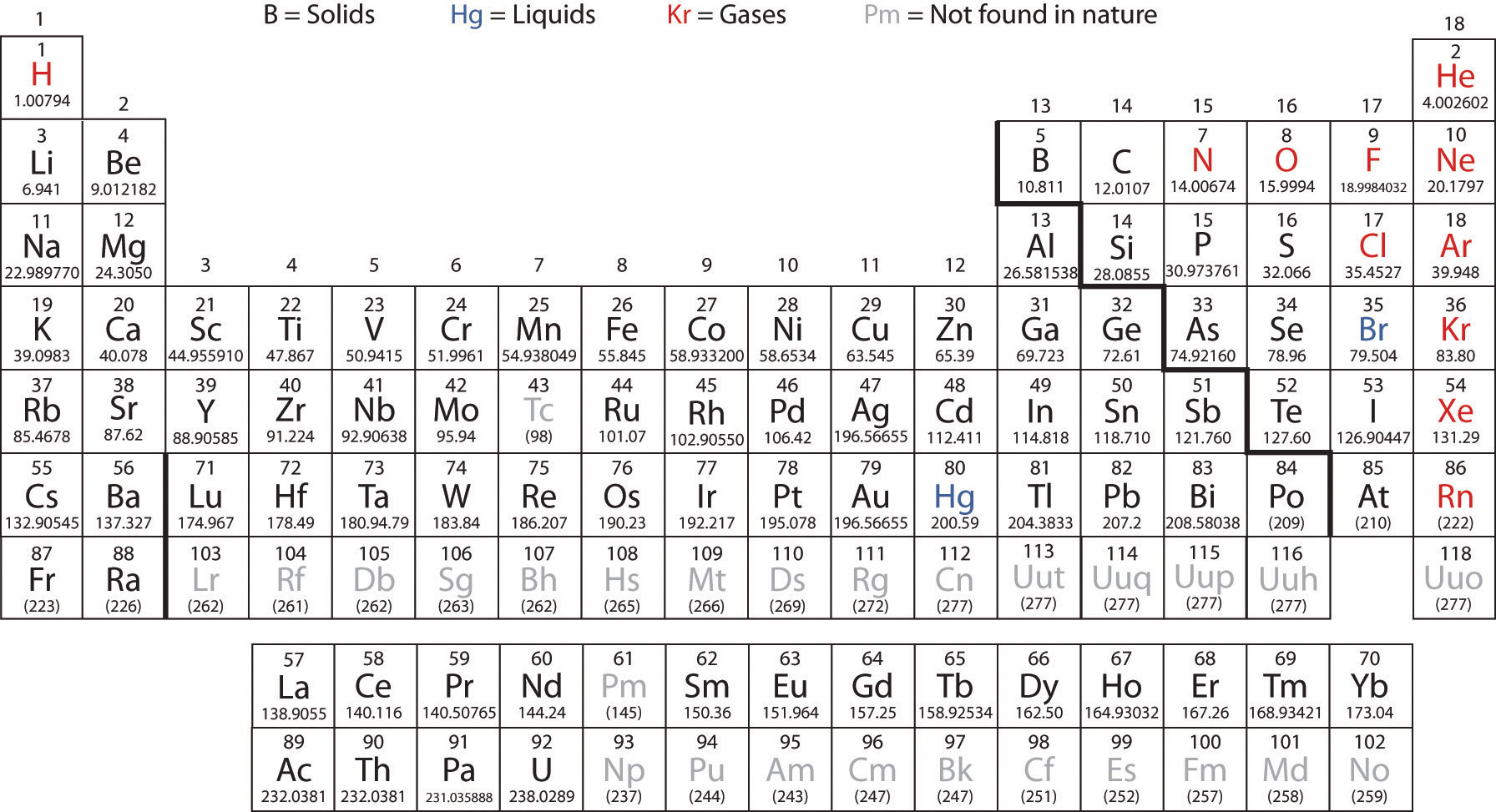
Fractional abundance of `text()^35 Cl =0. 7577`,
Molar mass `= 34 .9689`
Fractional abundance of `text()^37 Cl = 0.2423`,
Molar mass `= 36 .9659`
Average atomic mass
`= (0 · 7577) (34·9689 am u) + (0.2423) (36.9659 arn u)`
`=26.4959 + 8.9568 = 35.4527`
Fractional abundance of `text()^35 Cl =0. 7577`,
Molar mass `= 34 .9689`
Fractional abundance of `text()^37 Cl = 0.2423`,
Molar mass `= 36 .9659`
Average atomic mass
`= (0 · 7577) (34·9689 am u) + (0.2423) (36.9659 arn u)`
`=26.4959 + 8.9568 = 35.4527`